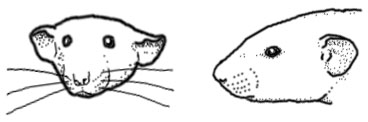
The dumbo rat mutation and human analogues
- Traits of dumbo rats
- Human analogues of the dumbo mutation
- What do these syndromes have in common?
- What are the first and second pharyngeal arches?
- Can a pharyngeal arch disorder be induced?
- Do other animals show first and second pharyngeal arch disorders?
- Is the dumbo mutation a disorder of first and second pharyngeal arch development?
- Update
Traits of dumbo rats
The dumbo mutation appeared first in the pet trade, and as of this writing the scientific community appears to be unaware of this interesting mutation in domestic rats. The dumbo mutation has not yet been noticed, measured or studied by developmental biologists or geneticists. No papers have yet been published on this mutation in rats.
Therefore, the following list of traits is entirely anecdotal. It has been gleaned from descriptions of owners of dumbo rats, and from descriptions of dumbo rat standards and faults from fancy rat organizations. This list is quite informal and imprecise. Not all dumbo rats have all of these traits:
Ear shape: The dumbo mutation produces a wide variety of ear shapes and sizes. Most desirable among pet rat breeders are large, round, low-set ears. But the dumbo mutation may also produce creased, bent, folded, wrinkled, curled, misshapen, narrow, pointed, oblong and tubular ears. Ears may be positioned higher or lower on the skull. Ears may also be asymmetrical.
Skull shape: The top of the skull may be flat and broad. Some dumbo skulls may be concave. Skull may have a prominent occiput (back of skull), which may give the rat a hunchback appearance.
|
|
Length/width |
Width/height |
Length/height |
|
Average |
1.75 |
1.07 |
1.88 |
|
Standard Deviation |
0.11 |
0.06 |
0.12 |
Dumbo rats
|
|
Length/width |
Width/height |
Length/height |
|
Average |
1.17 |
1.65 |
1.89 |
|
Standard Deviation |
0.22 |
0.27 |
0.19 |
From these tables, the length/height ratio appears to be fairly consistent between the standard and dumbo rats, but the dumbo heads appear to be relatively wider than standard heads. Sample measurements from one standard and one dumbo rat:
|
|
Length |
Width |
Height |
|
Standard |
56 mm |
32 mm |
30 mm |
|
Dumbo |
55 mm |
50 mm |
30 mm |
Note that these measurements are informal, and the number of individuals is too small to be able to draw conclusions about the population at large. However, it is an interesting first pass at head shape differences between standard and dumbo rats. Further study could be quite interesting.
Jaw shape: Some dumbos may have have a small lower jaw.
Eye shape: Some dumbos may show differences in eye shape and position (pers. comm. Dann 2005).
Body shape: Body may be stocky.
Ear movement: some owners report that their female dumbo rats do not vibrate their ears when they are in heat (pers. comm. Dann 2005).
Temperament: Dumbos are reputed to have a docile, calm temperament.
|
|
|
|
Human analogues of the dumbo mutation
There are several human disorders that are quite similar to the dumbo mutation in rats.
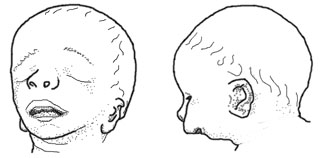
Treacher Collins syndrome: Characterized by small and otherwise deformed ears, a small jaw, downward-slanting eyes, and small cheekbones, a small face, and a wide mouth. The small jaw may force the normal-sized tongue back in the throat, obstructing the airway. Treacher Collins may also include hearing loss. It does not affect a child's intelligence. Caused by inherited or spontaneous mutations in the TCOF1 gene located at human chromosome 5tq31-q34 (Dixon et al 1991, Dixon 1996, Edwards et al. 1997, Horiuchi 2004) (OMIM entry)
Nager syndrome: Similar to Treacher Collins. Characterized by flat cheeks, downward-slanting eyes, absence of eyelashes, low-set, cup-shaped ears, and a very small lower jaw. May include malformations of the thumbs and forearms, genitourinary and cardiac anomalies (OMIM entry).
|
|
|
|
DiGeorge syndrome: Characterized by low-set ears, abnormal folding of the outer ear, small jaw, eyes slanted upward or downward, small upper lip, small mouth. Short stature and mild to moderate learning difficulties. Internally, characterized by a small parathyroid gland, small or absent thyroid gland, and cardiac malformations. Caused by deletions of chromosome 22q11.2 (Demczuk and Aurias 1995, Gong et al. 1996), which eliminates several candidate genes including TUPLE1 and Tbx1 (Jerome and Papaioannou 2001) ( (OMIM entry).
Goldenhar syndrome: Highly variable syndrome characterized by malformation or absence of the ears, anomalies of the middle and/or inner ear, small upper and lower jaw, small cheekbones, small lower skull, wide mouth, incomplete development of certain muscles of the face, small eyes, absence of tissue from the upper eyelid, and/or anomalies of the spinal column. Most cases are asymmetrical, with one side of the body more affected, and the other side unaffected or less affected. Most cases are spontaneous but some are inherited as an autosomal dominant (OMIM entry).
What do these syndromes have in common?
The syndromes listed above all belong to a subset of craniofacial disorders called pharyngeal arch disorders. All are caused by problems in the development of the structures derived from the pharyngeal (or branchial) arches.
What are the pharyngeal arches?
|
|
|
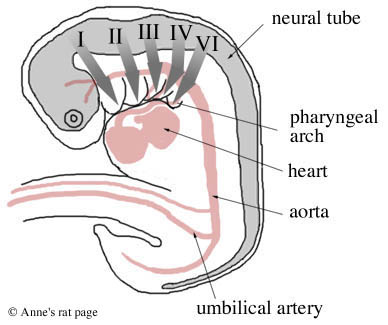
Diagram of the migration of neural crest cells (thick grey arrows) from the neural crest to the five pharyngeal arches (I, II, III, IV, and VI. Arch V degenerates). (Adapted from Gilbert 1994, p. 284.) |
Mammal embryos have five pairs of these pharyngeal arches. The first two pairs give rise to the bones, muscles, and nerves of the ear, jaw and upper neck. Specifically, the first pair of pharyngeal arches gives rise to two of the bones of the middle ear (incus and malleus), the lower jaw, and the nerves and muscles involved in chewing, and muscles of the ear and soft palate.
The second pair of arches gives rise to one bone in the middle ear (the stapes), most of the outer ear, the muscles of facial expression, muscles of the jaw and upper neck, parts of the bone above the larynx, and the seventh facial nerve. The last three pairs of arches give rise to the bones, muscles, and glands (thymus, thyroid) of the neck and the outflow tract of the heart.
Anything that disrupts the development of the first and second pharyngeal arches will cause parts of the face to develop abnormally. A disruption involves any disturbance of the production, growth, or movement of the arch cells during development -- for example, an insufficient migration of neural crest cells into the pharyngeal arches. A disruption could be caused by a mutation in one of the many genes involved in the development of these arches, or by external factors such as teratogens during pregnancy.
When the development of the first and second pharyngeal arches is disrupted, the arches develop abnormally, giving rise to a variety of craniofacial malformations: malformations of the external ear, ear canal, middle ear, cheekbone, upper jaw, lower jaw, eye, facial muscles, and nerves of facial expression (see also Jacobsson and Granstrom 1997).
|
Examples of genes involved in pharyngeal arch development TCOF1 is one of the many genes involved in pharyngeal arch development. Its protein, Treacle, is expressed most intensely in the first pharyngeal arch during times of critical craniofacial development, including the formation and fusion of the pharyngeal arches with the rest of the face (Dixon et al. 1997). Treacle is involved in ribosomal RNA production in prefusion neural folds during early embryogenesis (Valdez et al. 2004). Another such gene is TBX1, whose gene product has a wide variety of functions. It regulates proper neural crest cell migration in the posterior pharyngeal arches, stabilizes the structural patterns of the middle and inner ear during their development, regulates the onset of the development of jaw and neck muscles, controls the proper patterning of cells in the jaw, supports proper proliferation of cells fated to become part of the cardiac outflow tract, and is required for the formation of the division between the aorta and the pulmonary artery (Moraes et al. 2005, Kelly et al. 2004, Xu et al. 2004). |
Can a pharyngeal arch disorder be induced?
Yes.
Several teratogens can induce low-set ears and other ear malformations in rats, mice and hamsters. Retinoids (such as retinoic acid and vitamin A), cyclophosphamide, and isotretinoin (Accutane) administered during pregnancy give rise to a suite of anomalies of the ear, eye, upper and lower jaw, and palate, resembling Treacher Collins and other first and second pharyngeal arch syndromes (Emmanouil-Nikoloussi et al. 2000, Granstrom 1990, Granstrom and Kirkeby 1990, Granstrom et al. 1991, Jarvis et al. 1990, Mallo and Gridley 1996, Padmanabhan and Singh 1984, Poswillo 1975, Wiley et al. 1983).
Diabetes mellitus during pregnancy may also cause congenital malformations in the young due to impaired development of cranial neural crest cells. Such malformations include low-set ears, small jaw, small thymus, thyroid, and parathyroid glands, and anomalies of the heart and great vessels. These malformations are very similar to DiGeorge syndrome in humans (Siman 1997, Siman et al. 2000).
Do other animals show first and second pharyngeal arch disorders?
Yes.
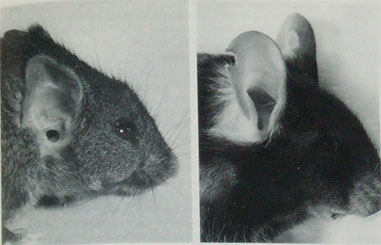 Right ear of an eight month old LSE+ mouse (left) and a control mouse (right). The notch in the control mouse's
ear is an ear mark. From Theiler and Sweet 1986.
Right ear of an eight month old LSE+ mouse (left) and a control mouse (right). The notch in the control mouse's
ear is an ear mark. From Theiler and Sweet 1986.
Otani et al. (1991) report the spontaneous appearance of small ears, some of which were low-set, in a line of transgenic mice. The disorder was asymmetric. Affected mice also had disorders of the middle ear, cranial base, upper jaw, and pharyngeal structures. The disorder was heritable as an autosomal dominant, homozygous lethal. The ear anomaly was traced to underdevelopment of the second pharyngeal arch during the 9th and 10th days of gestation, due to mesenchymal disruptions and hemorrhage in the region of the first and second branchial arches. The disorder was traced to a mutation on chromosome 10, B1-3, at a site named Hfm (Cousley et al. 2002, Naora et al. 1994).
Dogs: Haworth et al. (2001) studied TCOF1, the homologue of the Treacher Collins gene in humans, in 13 different dog breeds. They discovered that TCOF1 has nine different variations in dogs. One of these variants (C396T) is associated with a broad skull and short face (brachycephaly) in dogs. The authors suggest that this mutation may have arisen only once in the history of dog domestication.
Is the dumbo mutation a disorder of first and second pharyngeal arch development?
Nobody knows for sure, because the dumbo mutation has never been studied in the laboratory.
However, there are strong similarities between the features of dumbo rats and the clinical features of first and second pharyngeal arch syndromes in humans. The dumbo rat's malformed and malpositioned ears, possible small jaw, possible differences in eye shape and position, and possible underdevelopment of the muscles that move the ears or the nerves that control them, are all strongly suggestive of a first and second pharyngeal arch disorder.
This is a hypothesis at this point, of course. The answer must await future investigation by geneticists and
developmental biologists. Dumbo rats may turn out to be a potential animal model for first and second pharyngeal
arch disorders in humans.
March 2005
Update 2010
Update, 2010: It looks like the dumbo mutation is indeed a disorder of first pharyngeal arch development!
Katerji et al. (2009) examined the craniofacial development of Dumbo rats. They found that Dumbo rats show quantitative defects in the structures derived from the first pharyngeal arch: disturbances in the development of cartilage and the beginning of ossification.
They examined the expression of the Msx1 and Dlx1 genes in the Dumbo rat during craniofacial morphogenesis. The Msx and Dlx homeobox genes are expressed at different times and in several different locations of first pharyngeal arch development, where they perform a variety of roles.
The authors found that Msx1 and Dlx1 are expressed significantly less during Dumbo rat than control rat morphogenesis, indicating that these genes may underly the Dumbo phenotype.
Further reading
- Branchial anomalies, from the Texas Pediatric Otolaryngology Center
- First and second pharyngeal arch syndromes, by Foundation for Faces of Children.