It is one of the most distressing and incomprehensible behaviors a human can witness: a pet rat killing infant rats, a mother consuming her own offspring. Humans today have a such a strong taboo against killing human infants, their own or those of other people, that such behavior in a pet is appalling.
Why would an animal do such a thing? A great deal of research has been done on infanticide in rats, and this article examines why adults might kill infant rats, and what factors influence infanticidal behavior.
Summary
Infanticide, or pup-killing, is found in many species, including rats. In rats, the infanticidal animal may be the mother, a strange male, or a strange female. Each of these may commit infanticide for different reasons. Most infanticide is directed at newborn rats.
Mothers tend to kill deformed or wounded infants, which may allow her to allocate resources to the healthy pups who are more likely to survive. Mothers may also kill entire litters when they are stressed, perhaps because the mother perceives the environment as too hostile for pup survival, or she perceives herself as unable to rear the litter successfully, so she recuperates some her energetic investment by consuming the young. Malnourished mothers, and mothers who have an abnormal birth experience, may also become infanticidal.
Unrelated adult male rats may kill young in order to bring the mother back into estrus sooner and thus hasten the arrival of a litter of his own. To keep from accidentally killing his own young, infanticide toward all infants is reduced in males from 18-50 days after copulation, a time that roughly corresponds to the period from birth to weaning of their own offspring. Infanticide is also reduced by cohabitation with a pregnant female. A chemical produced by pregnant females may suppress infanticide, and maternal aggression after the birth may also play a role in preventing male infanticide, though its success is mixed. Repeated exposure to young also inhibits infanticide in males, and generates parental behavior.
Unrelated females commit infanticide to gain a food source by consuming the litter, and to take over the nest of the destroyed litter. As with unrelated males, infanticide in unrelated females can be reduced by cohabitation with the pregnant female and by exposure to pups. Relatedness and familiarity also play a role: pregnant sisters who have lived together since birth are rarely infanticidal toward each other's litters, and often participate in cooperative rearing. In contrast, nearly half of pairs of unrelated pregnant females who cohabit just during their pregnancies experience infanticide, and cooperative rearing is less common.
What is infanticide?
Broadly defined, infanticide means killing young of one's own species. It is sometimes followed by cannibalism, which means eating all or part of an individual of the same species (e.g. Zarrow et al. 1972). Infanticide has been found in many species, including humans, primates, felines, canids, cetaceans, rodents, insects and fish, and has generated a large body of literature (for reviews, see Ebensperger 1998, Hausfater and Hrdy 1984, Labov et al. 1985, van Schaik and Janson 2000).
Among rodents, rats aren't the only ones who commit infanticide. Infanticide has been recognized in many species of rodents: Belding's ground squirrels (Sherman 1981), California ground squirrels (Trulio et al. 1986), Collard lemmings (Mallory and Brooks 1978), Columbian ground squirrels (Waterman 1984), golden hamsters (Marques and Valenstein 1976, Richards 1966), house mice (McCarthy and vom Saal 1985), meadow voles (Webster et al. 1981), Mongolian gerbils (Elwood 1977), muskrats (Errington 1963), prairie dogs (Hoogland 1985), white-footed mice (Wolff 1986), and yellow-bellied marmots (Brody and Melcher 1985)
Why commit infanticide? Infanticide may be committed for a variety of reasons. Many of these reasons indicate that infanticide may be adaptive to the killer. Animals may commit infanticide in order to:
- gain a food resource by consuming the young (the predation hypothesis).
- gain increased access to physical resources like food, nesting sites, or space (the resource competition hypothesis).
- avoid adopting and providing providing care to unrelated offpsring (the adoption avoidance hypothesis).
- bias the sex ratio of the litter
- Adult males may kill a female's young in order to increase his own chances of mating (the sexual selection hypothesis).
- Lastly, infanticide may be neutral or maladaptive (pathological) and may be the product of selection for another type of behavior like aggression, or may be an accident, or may be the result of disturbances in the physical or social environment (see Ebensperger 1998 for review).
Infanticide in rats
Who commits infanticide? Infanticide is multifaceted. In rats, the infanticidal animal may be the mother, a strange male, a father, or a strange female. Each of these may commit infanticide for different reasons under different circumstances.
Who is the target of infanticide? Newborn pups (1-3 days old) are far more likely to be killed by adults than 10-12 day old pups, and weanlings (21-25 day old) are almost never killed (Mayer and Rosenblatt 1980, Paul and Kupferschmidt 1975).
The killing of deformed, sick, and weak pups and the consumption of already-dead ones are different from the killing of healthy offspring. Lastly, the consumption of an entire litter of pups by the mother may be different from the ingestion of part of a litter followed by good care of the remaining offspring (DeSantis and Schmaltz 1983).
Description of infanticide. Weanling rats are more likely to be killed by predatory attack. The adult pounces on the young rat, the adult's head repeatedly lunges downward and bites the young animal on the back and neck, especially the base of the neck, severing the spinal cord (Paul and Kupferschmidt 1975). This attack is the same one rats use to kill mice.
Ten-day-old pups are killed with inconsistent technique that may be predatory or hold-and-eat. Sometimes they are killed with a well-directed predatory biting attack or a pounce-and-bite with bites directed at the back or legs (and not the base of the neck). Sometimes ten-day-old pups are attacked with eating bites in which the adult grips the pup with its teeth and chews and grinds. Newborn pups are simply eaten. The adult rat picks the pup up in its paws and nibbles on it as if it were a food item (Paul and Kupferschmidt 1975)
Therefore, the killing of older pups is predatory, but the killing of infant pups is accomplished simply through eating (Paul and Kupferschmidt 1975, Peters and Kristal 1983).
Newborn rats appear to taste good to rats. Adult rats who had previously killed neonatal rats readily consumed large amounts of them (18.5 grams in two hours), even though the adults' normal food was present. Taste, odor, lack of movement, and lack of hair may all play a role in making a dead neonatal rat an attractive food item (Paul and Kupferschmidt 1975).
Infanticide by mothers
Most maternal cannibalism occurs in the first 24 hours, and tapers off steeply during the first week (Schardein et al. 1978, DeSantis and Schmaltz 1984, Reynolds 1981). However, in rare cases a mother may kill older offspring (e.g. Babicky and Novakova 1986 found that some malnourished mothers killed weanling pups).
Causes and functions of maternal infanticide
Infanticide of malformed pups: Females who give birth to deformed young are more likely to consume the deformed offspring than their healthy siblings (called culling), and are more likely to eat already dead young (whether deformed or healthy) than live ones (Reynolds 1981).
Schardein et al. (1978) found that 21% of live congenitally malformed offspring were killed and consumed, contrasted to only 3% of normal live offspring. Regarding stillborn offspring, mothers consumed 63% of the congenitally malformed ones, and 19% of the normal ones. When the entire litter was alive and normal, there was no cannibalism at all, but most of the litters containing some malformed pups experienced infanticide. Which infants were cannibalized was variable, however: in one case, a female killed a normal pup and left the malformed one, in another case a female killed one malformed pup but left the others who had the same defect, and in three cases the females consumed the entire litter.
Newborn rats who are wounded may also be killed (Helander and Bergh 1980). DeSantis and Schmaltz (1984) found that the more wounded the pup, the more likely it was to be killed by the mother. The mothers then proceeded to provide good care to the remaining offspring.
The killing of malformed offspring seems adaptive, as it permits females to adjust the final composition of their litters based on environmental and physiologal conditions at birth. By removing the pups who are least likely to survive, the mother can give more resources to the remaining pups, increasing their chances of survival, and she can recover some proportion of the resources allocated to the malformed pups through cannibalism.
Stress-induced infanticide: Stress also plays a role. Physical factors such as excessive noise, rough handling, and too frequent cage cleaning or movement were advanced as possible causes of maternal cannibalism by Lane-Petter (1968).
DeSantis and Schmaltz (1984) mention two cases of entire-litter cannibalism in response to stress: one case involved a female who was transported during her pregnancy who cannibalized her litter during birth. Another was a female whose cage was accidentally flooded with water from a broken water bottle soon after birth, soaking the bedding and wetting the pups. The pups were subsequently consumed within 24 hours.
Stress-induced infanticide may be aberrant. However, one possible adaptive explanation is that that if the mother perceives the environment to be too hostile for pup survival she may give up supporting them further, and may recuperate some of her energetic investment by consuming the litter.
Infanticide from maternal malnourishment: Several studies have examined diet and infanticide. Obesity has been found to increase the frequency of infanticide and cannibalism in rats. Wehmer et al. (1979) found that only 48% of obese females who were kept on a high fat diet gave birth (compared to 100% of normal females), and of these obese mothers 55% cannibalized their young within the first week.
Females who were fed too little protein (e.g. 5-6% protein in diet) after giving birth tended to have slower-growing offspring who were weaned later than those of normally fed mothers, and some of these malnourished mothers committed infanticide during the weaning period (Babicky and Novakova 1986). Females fed on a vegetarian diet were far more likely to cannibalize their pups than females fed an omnivorous diet (Carlson and Hoelzel 1948).
Vitamin defficiencies are also associated with infanticide and cannibalism: 39.7% of females fed a diet deficient in vitamin B12 cannibalized their litters, compared to only 20.5-23.3% of females whose diets contained vitamin B12 (15-30 µg/kg) (Hankin 1960).
Infanticide of pups of one sex: Under stressful conditions, mother rodents who live in polygamous social systems may selectively kill their male offspring.Why? Because in polygamous systems an adult's chances of breeding are very different for females and males. A female is highly likely to breed and produce offspring. A male, however, has to become socially dominant in order to have the best chance at impregnating females. Therefore, while females can produce far fewer offspring than males can potentially sire, in polygamous systems a female's reproduction is nearly assured, but only a subset of males ever become fathers.
A mother whose pregnancy occurs under stressful conditions (e.g. not enough food) is likely to produce a litter of unthrifty pups who will grow into undersized adults. Undersized daughters are still likely to breed when they grow up, but undersized adult sons may never breed at all. Therefore, it may be adaptive for stressed mothers to kill their male pups.
The killing of male pups by stressed mothers has been found in mice (Rivers and Crawford 1974), hamsters (Huck et al. 1983), and wood rats (McClure 1981), but has not yet been demonstrated in Norway rats.
Note that Norway rats can also bias the sex ratio of their litters in utero. For example, Norway rats who become pregnant right after the birth of one litter (during postpartum estrus) but lose their current newborn litter tend to give birth to female-biased litters. If their current litter survives and nurses, however, the subsequent postpartum-conceived litter contains an equal number of males and females (Bacon and McClintock 1994, 1999).
Abnormal birth experience: Females who give birth prematurely (24 hours early) (Vickery 1979) or by Cesarian section (Stern 1985) are more likely to kill their young than females who give birth normally (specifically, 21.9% of mothers who delivered by c-section committed infanticide, vs. none who delivered normally, Stern 1985).
Infanticide after Cesarian section could be due to the moribund condition of the pups, many of which die soon after delivery. Also, Cesarian-delivered pups are typically delivered on day 21 of gestation, instead of day 22. They tend to be smaller and may lack the same sensory quality as pups who experience an additional day of gestation and a natural delivery (Peters and Kristal 1983).
A premature birth or c-section could lead to infanticide because the normal hormonal profile that accompanies normal full-term labor and delivery and helps generate maternal behavior is disrupted by premature birth or c-section. For example, the stimulation of the passage of young through the birth canal may be important for triggering subsequent normal maternal behavior.
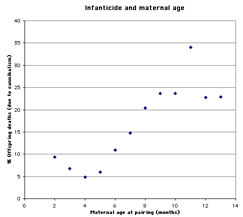
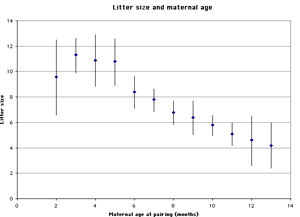
Infanticide and age of the mother: Mohan (1974) studied infanticide, litter size, and maternal age in 240 female rats. The author found that very young mothers and especially old mothers were more likely to cannibalize their offspring than mothers of intermediate age (figure 1). The size of the litter follows an inverse pattern: very young and especially older mothers tend to have smaller litters than those of intermediate age (figure 2).
|
Figure 1. Infanticide and maternal age (graph based on data from Mohan 1974) |
Figure 2. Litter size and maternal age (graph based on data from Mohan 1974). Error bars refer to one standard deviation. |
Infanticide by unrelated adults
Adult, unmated rats of either sex do not normally show parental behavior toward strange infants. Adults tend to approach them tentatively, sniff them, and withdraw quickly. Generally, adults ignore them (Rosenblatt 1967), but sometimes adult females (Peters and Kristal 1983), and especially adult males, attack and kill newborns.
In general, male rats are more likely to kill strange pups than females, but rates of infanticide vary with the rat's strain and sometimes with the laboratory in which the rats are raised (Brown 1986).
- Wistar rat males tend to be infanticidal while females show low rates of infanticide: Jakubowski and Terkel (1985a), found that 76% of males and less than 10% of females killed pups. Rosenberg et al. (1971, 1974) found that 56.7% to 79.5% of males killed strange newborns, compared with 9.8% of nulliparous females (females who had never given birth). Fleming et al. (1980) found that 15.4% of Wistar females are infanticidal.
- Long-Evans rats were similar to Wistars, with more infanticide in males than females: most studies found no infanticide in females but up to 62% of males were infanticidal (Miley et al. 1982, Miley et al. 1981, Paul and Kupferschmidt 1975). Similarly, Brown (1986) found that 91.9% of males and 16.7% of females were infanticidal. However, Peters and Kristal (1983) found that 80% of virgin Long-Evans females killed newborn pups and that pregnancy suppressed infanticide in these females.
- Sprague-Dawley rats are more variable. Some studies show that, as with Wistar and Long-Evans rats, males are more infanticidal than females. Bridges (1983) found that no females were infanticidal but 53% of the males were. However, others studies have found higher rates of infanticide in females: Fleisher et al. (1981) found that 30% of females and 53% of males were infanticidal (see also Mayer and Rosenblatt 1979). On a different note, several studies have found that infanticide is low in both males and females. Jakubowski and Terkel (1985a) found that less than 6% of males and females were infanticidal (see also Mayer et al. 1979).
Newborn pups (1-3 days old) are more likely to be killed by adults than 10-12 day old pups, and weanlings (21-25 day old) are almost never killed (Mayer and Rosenblatt 1980, Paul and Kupferschmidt 1975).
In pregnant females, non-parental behavior is replaced by parental behavior at the time of birth, and females display maternal behavior such as licking, pup retrieval, and hovering over the pups in the nursing posture (Nelson 1995, Peters et al. 1991).
Infanticide by unrelated adult males and fathers
Infanticide is not a "fixed" trait in males. It is modified by a number of factors, such as testosterone levels, litter effects, and copulation and cohabitation with a pregnant female.
The presence of testosterone in the young male rat is implicated in infanticidal behavior. Male rats castrated while young are much less likely to kill newborn rats than intact controls (32% of castrates kill strange newborns, compared with 79.5% of intact males), but if they are given replacement injections of testosterone their infanticidal behavior resembles that of intact rats (76% are infanticidal: Rosenberg et al. 1974).
There also appears to be a litter effect in some cases, with infanticide running in litters. Brown (1986) found that all males from some litters were infanticidal, while no males from other litters showed infanticide. These litter differences could be genetic, or due to maternal effects, or to post-weaning rearing conditions (because litters in this laboratory lived together even after weaning). One way to examine this effect further would be to conduct split litter or cross-fostering experiments.
Why do unrelated adult male rats commit infanticide?
Hrdy (1979) proposed that the adult males kill infants in order to bring the mother back into estrus more quickly so he can mate with her and produce offspring of his own. Also, destroying an unrelated litter reduces the number of immature animals who might compete with the killer's own offspring for resources. Therefore, killing the infants of other males enhances a male's reproductive success (for more discussion of this topic, see van Schaik and Janson 2000).
Males tend to kill litters that are not theirs: Mennella and Moltz (1988) housed sexually naive males with pregnant females. Most of the males (4 out of 6) were infanticidal after the birth of the pups. The mother attempted to defend her young, by hiding the pups and by biting and clawing the male. The mothers were unsuccessful and the males killed and usually cannibalized the entire litters. Then the male mated with the female himself.
In rats, however, females have a "postpartum estrus." The timing of this estrus depends on both the time of day and the time elapsed since the birth. A female rat tends to come into heat the first evening that is at least 10 hours after the birth (Gilbert et al. 1985).
Therefore, one could argue that a male need not destroy a female's new litter in order to mate with her and sire a litter of his own. However, killing a new litter may still benefit the male by preventing a lactation-induced delay in implantation. Eggs fertilized during a female's post-partum estrus do not implant right away (Mantalenakis and Ketchel 1966). By killing a new litter and mating during the female's postpartum estrus, the male's offspring are born after the normal 21-22 days of gestation rather than after an extended gestational period.
Mennella and Moltz (1988) found that males who destroyed a newborn litter by another male and mated with the female during her post-partum estrus saw the birth of their own young 23 days later. In contrast, males who allowed the litter to survive saw the birth of their own young 32 days later (+- 1 day).
After the postpartum estrus, the female does not come into heat again until after weaning. Therefore, if a male misses the postpartum estrus he can advance the female's next estrus by killing the current litter and terminating lactation. The female then enters estrus again within 6 days, and the infanticidal male mates with her and sees the birth of his own litter about 23 days later. Males who leave the female's offspring alone after postpartum estrus, however, have to wait for the female's post-weaning estrus which occurs about 29 days after partuition. Such males have to wait about 50 days for the birth of their own litter (Mennella and Moltz 1988).
Rats have lifespans of less than one year in the wild (Davis 1948). Waiting a month for a female to come into heat while she nurses an unrelated litter is therefore a poor strategic choice for a male. He can increase his reproductive fitness by destroying the female's current litter and quickly siring one of his own.
Inhibiting infanticide in males
A problem with infanticide by adult males is that a male might kill his own offspring. A male who promotes the conception of a new litter by killing a litter he has already sired has confounded himself. How, then, do male rats keep from killing their own young?
One possibility is that a father might recognize his own offspring, but so far, there is little evidence to support this kind of kin recognition in adult male rats.
Instead, it appears that infanticide in male rats is suppressed at times when their own offspring might be young and vulnerable, as it is in male mice (vom Saal 1985). Mennella and Moltz (1988) found that copulation reduces male infanticidal behavior starting several weeks later, at a time that roughly coincided with the birth of the male's young (see also Brown 1986). This inhibition generalized to all pups: not only did males refrain from killing their own pups, but they tended not to kill the pups of other males, either. Infanticide came back to normal levels about 50 days after the male mated, a time which roughly corresponds to the weaning of the pups. Therefore, male infanticide toward pups may be reduced at a time when his own offspring are vulnerable to infanticide. Interestingly, it has been suggested that females may exploit copulation-induced infanticide suppression by mating with many males in a short span of time, thus inhibiting all of them when her young are born (e.g. in mice, Huck et al. 1982). To date, no studies have examined this possibility in rats.
Cohabitation with a pregnant female also inhibits infanticide. The longer males live with females during pregnancy, the fewer males were infanticidal. This "cohabitation effect" is not specific to the female a particular male lives with: the male is inhibited from killing the young of other, unfamiliar females, too (Mennella and Moltz 1988). Conversely, cohabitation with a non-pregnant female has no effect on infanticide (Brown 1986).
Sexual experience and cohabitation combined almost completely inhibit infanticide in known pup-killing males: after mating with a female and living with her through the pregnancy, lactation, and pup-rearing, known pup-killing males were presented with unfamiliar newborns they had not sired, and only 8.3% (1 out of 12) committed infanticide. This was not a temporary phenomenon: 60 days later, the males were tested again and none were infanticidal (Brown 1986).
The cohabitation effect may be due to a chemical signal emitted by the female during pregnancy which inhibits infanticide in males, because prolonged exposure to soiled bedding from a pregnant female also inhibits infanticide in males (Mennella and Moltz 1988, see also 1988b).
The mother may help prevent infanticide by a male through maternal aggression. Female mice may "condition" the male to avoid the pups when they are born (Hedricks and Daniels 1981). In an experiment on parental behavior in male rats, it was found that 7 out of 8 males who lived with lactating females had wounds on their front paws or tails, while no females were wounded (Brown 1986b). However, maternal aggression shortly after birth is frequently unsuccessful in thwarting a male who is intent on destroying a newborn litter (e.g. Mannella and Moltz 1988, Erksine et al. 1978). Also, female aggression peaks at 9 days after the birth of the litter (Erskine et al. 1978), while most infanticide occurs within a few days of birth.
Repeated exposure to pups alone may also stop male infanticide: infanticidal males who are repeatedly exposed to foster pups (called concaveation) eventually stop killing them, and start behaving parentally (Jakubowski and Terkel 1985b). In fact, after 6-7 days of exposure, males may start to show maternal behaviors such as nesting, licking the pups, and hovering over them in the nursing posture (Rosenblatt 1967).
Infanticide by unrelated adult females
Female rats who have never given birth (nulliparous) may kill newborn rats (Peters et al. 1991). Female rats kill unrelated young usually when they are sexually inexperienced, pregnant, or after weaning their own litters, but rarely when they have a young litter of their own (Peters and Kristal 1983). Hence, females commit infanticide only when unrelated offspring can be clearly distinguished from their own.
Why do unrelated adult female rats commit infanticide?
Predation and nest theft: Infanticide may benefit the nulliparous females because they obtain food through cannibalism of the young, and can take over the nest of the destroyed litter.
Menella and Moltz (1989) allowed unfamiliar pairs consisting of one nulliparous and one pregnant female to live together for 5-10 days before the birth of the pregnant female's litter. Fifty-five percent of the nulliparous females exhibited infanticide shortly after the birth, and almost all of the killed pups were cannibalized. And, within three days of the birth and destruction of the litter, the nulliparous female had moved into the nest and displaced the mother of the destroyed young. Nulliparous females who did not commit infanticide never took over the mother's nest.
Inhibiting infanticide by adult females
How, then, are nulliparous females prevented from killing the offspring of others? As with males, cohabitation with the pregnant female reduces infanticide in the nulliparous females. Nulliparous females who live with pregnant females for the duration of the pregnancy are unlikely to commit infanticide (9% are infanticidal as opposed to 55%, see above). In addition, 60% of these females behaved maternally after about 3.5 days, licking, retrieving, and crouching over the pups (Mennella and Moltz 1989).
Exposure to pups also reduces infanticide and induces maternal behavior in nulliparous females: after several days (6-8) of exposure to pups females may start to build a nest, retrieve young, and huddle over them in the nursing posture (Jakubowski and Terkel 1985b, Rosenblatt 1967, Wiesner and Sheard 1933).
As with males, the "cohabitation effect" may be partly due to a chemical signal synthesized by the mother. This chemical appears to inhibit infanticide in nulliparous females, and even generates maternal behavior in some of them (Mennella and Moltz 1989).
Related females and infanticide
Relatedness and familiarity between the females play a role as well. In a communal rearing situation, in which pregnant females live and raise their litters together, Schultz and Lore (1993) found that sisters who had been raised together were far less likely to exhibit infanticide toward another's pups than unrelated females who cohabitated just during their pregnancies (11% of sister pairs experienced infanticide, compared to 44% of unfamiliar pairs of females). In almost all the unfamiliar pairs, infanticide consisted of one pregnant female killing the newly-born litter of the other female before giving birth herself a few days later.
The pup's strategy
Pups are not completely helpless when faced with a unfamiliar adult rat. Pups tend to stop moving and make fewer sounds when they smell an unfamiliar, adult rat (Takahashi 1994, Wiedenmayer and Barr 2001). This response wanes around weaning (about day 26) (Wiedenmayer and Barr 2001) when the rats are at much lower risk of being killed by unfamiliar males (e.g. Robitaille and Bovet 1976).