Where do Rat Coat Colors Come From?
Rats come in a wide variety of colors and patterns, from solid black to white, from dark browns to warm tans to creams, from slate grey to pale blue. Rats can display patches of white that range from a small chest spot to a belly blotch to white with a pigmented head and beyond. The variation is immense! Where do these coat colors and patterns come from? How are the different pigments made and integrated into the growing fur? How do mutations along the pigment production pathway produce the different colors we see in rats?
- A pigment primer
- Mutations in pigment cell distribution
- White patches and spots: hooded, spotting lethal, and white spotting mutations
- Mutations in melanosome formation
- Mutations in pigment transport and deposition
- Mutations in pigment synthesis
- Albino and siamese: mutations in the chinchilla gene
- Pink-eyed dilution
- Brown
Disclaimer: I am a biologist, not a rat breeder, and my interest in rat colors stems from my fascination with the biology of pigmentation. Therefore, this is not an article about rat standards! If you are seeking rat color standards, descriptions, and their associated Mendelian notations, check out one of the many websites of fancy rat organizations such as AFRMA, RMFE, NFRS, and AusRFS to name just a few.
Not all rat colors are included here because I have focused my attention on the mutations whose cellular consequences are known. There are many other colors whose specific causes are not yet known.
A word about color names... I have occasionally included color names for purposes of illustration. Note, however, that such color names may vary between rat organizations and countries. The same color may be called by different names; a particular name may refer to different colors, depending on who you talk to. Lastly, a particular color may have a number of possible underlying causes, so use caution when extrapolating from this document to your pet rats. By and large, I have tried to steer clear of "official" color names and rat standards, and have instead focused on my area of interest: the biology of pigmentation.
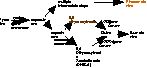
Pigments are made in cells called melanocytes. During development of the embryo, the cells that will become melanocytes (these precursors are called melanoblasts) migrate from the dorsal area -- from an active region running along the back of the embryo called the neural crest -- to the rest of the body. These cells take up positions at the base of hair follicles. They also become integral parts of other organs such as the eye, inner ear, and other neural systems (Figure 1). If the migration of these cells is disrupted, then some areas of the body won't have melanocytes, and these areas of the body will produce white hairs.
|
|
Figure 1. During development of the embryo, melanocyte precursors migrate from the neural crest. These neural crest cells differentiate into many different cell types, including melanocytes. Disorders in melanocyte migration and differentiation include hooded, spotting lethal, and white spotting. |
The melanoblasts differentiate into melanocytes, which live in the skin and at the base of each hair, and produce melanin pigments. The menanin pigment particles, called granules, are incorporated into the growing hair (Figure 2).
|
|
Figure 2. Cross-section of a hair and follicle. Melanocytes synthesize melanin granules which are incorporated into the growing hair (click on image for larger picture). |
Inside the melanocyte...
Pigment particles, called granules, are synthesized in little vesicles inside melanocytes. These vesicles are called melanosomes. Two types of pigment are produced in melanosomes: yellow-red pigments called phaeomelanins and brown-black eumelanins.
There are actually separate melanosomes for the two types of pigment. Phaeomelanosomes specialize in making the yellow-red phaeomelanins. Eumelanosomes specialize in making the brown-black eumelanins.
A melanocyte may switch between producing pale and dark pigments in a single hair. In this case the effect is banded hairs, as seen in the agouti allele.
Melanosomes produce pigments inside the melanocyte. Melanosomes start their journey in the middle of the melanocyte cell, and migrate to the outer edge of the cell, through projections called dendrites (Figure 3).
[Melanocytes are actually a type of neural cell, coming from the neural crest, so they have some neuron-like properties such as dendrites].
At the outer edge of the cell, melanosomes release their pigment, which is then incoporated into the surrounding keratinocytes and the shaft of the growing hair.
|
|
Figure 3. Melanocytes contain melanosomes: phaeomelanosomes and eumelanosomes, which synthesize pale and dark pigments. Switching between pale and dark pigment production on a single hair produces banded hairs, called agouti. Melanosomes migrate to the base of the dendrites. From there they are transported to the dendrite tips. Disorders in melanosome formation in rats include red-eyed dilution. |
How do melanosomes migrate from the middle to the edge of the melanocyte? Melanosomes are actually attached to a framework of microtubules, and are transported up the dendrites on ladders of actin. The little "feet" that walk up the actin fibers are called myosin 5 (Figure 4).
|
|
Figure 4. Melanosomes are attached to a branching microtubule arbor inside the melanocyte. To move up the dendrite, they attach to actin filaments with myosin 5, and the myosin 5 "walks" them to the cell edge (click on image for larger picture). Disorders in melanosome transport include the dilution allele. |
Inside the melanosome...
Inside the melanosomes, mammals make two kinds of pigments: eumelanins, which range from dark brown to black, and phaeomelanins which range from red to yellow.
Both types of pigment are made from the same starting product, the amino acid tyrosine, and the first several steps of their production are the same.
The enzyme tyrosinase (coded by a gene called chinchilla) converts tyrosine, which is colorless, into dopaquinone, also colorless. Phaeomelanins are made out of these.
To make eumelanin, dopaquinone is converted into dopachrome. Dopachrome can take two pathways to eumelanin. In the first pathway, dopachrome is converted into 5,6 dihydroxyindole, which is brown, and then into complex quinone compounds by the enzyme tyrosinase related protein I (TYRP1, produced by the brown gene). In the second pathway it is converted into DHCA by dopachrome tauromerase (DCT) and from there to complex quinones by TYRP1 again. Complex quinones are then polymerized into eumelanin, which is black (Figure 5).
|
|
Figure 5. Simplified synthesis of the melanins phaeomelanin and eumelanin. Disorders in pigment synthesis include chinchilla (codes tyrosinase) and brown (codes TYRP1). (click on image for larger picture). The tradeoff between synthesis of phaeomelanin and eumelanin along the shaft of a growing hair is mediated by the agouti allele. |
Phaemelanosomes and eumelanosomes are actually quite different inside. Phaeomelanosomes are spherical in shape and are quite primitive, lacking TRP1, TRP2, and p-protein (seen in pink-eyed dilution). They have only one third the level of tyrosinase as eumelanosomes.
Eumelanosomes are more sophisticated, oval-shaped melanosomes, which have TRP1, TRP2, p-protein, and three times the tyrosinase as phaeomelanosomes.
Conclusion
Variations in hair color can arise from differences in synthesis of pigment, resulting in black, brown, yellow or colorless pigment granules, or in deposit of pigment in the hair shaft. Differences in pigment deposit in the hair produce variation in the intensity and shade of the color. The color and amount of granules can vary along the length of a single hair, or between different hairs. Variation in pigmentation comes from patterns of melanocyte migration and final distribution. Areas with melanocytes become pigmented, but areas without melanocytes produce depigmented, white hairs.
In the next sections I'll examine many different mutations occuring along this pathway, and how these mutations give rise to the final coat color. I'll also examine other "side" effects of these mutations, and possible parallels found in human pigmentation.
Mutations in Melanocyte Distribution
A delay in melanocyte migration: the hooded alleles, spotting lethal, and white spotting alleles
Introduction
In the embryo, a fold develops down the back called the neural tube, which contains an active region called the neural crest. This region supplies the pigment cells (melanocytes) that migrate all over the body.
Specifically, the pigment cells migrate to pairs of specific sites on either side of the body as well as the backline. There are three such sites on the head (near the eye, near the ear, and near the top of the head), and six sites along each side of the body, and several along the tail. A few pigment cells migrate to each of these sites, where they proliferate and migrate outwards, joining up to form larger patches, spreading down the legs and down the head until they meet up under the chin, and down the body until they meet up on the belly (Cattanach 1999).
Once the pigment cells have finished migrating they take up positions at the base of hair follicles. There they synthesize melanin pigment, and feed it into the growing hair. Normally, all follicles have pigment cells associated with them and all the animal's fur is pigmented. But if no pigment cells are associated with a follicle, there is no pigment in that hair. Mutations that affect pigment cell distribution during the development of the embryo determine which parts of the body have pigment cells, and hence produce pigment, and which parts have no pigment cells and produce depigmented hairs.
Pigment cells also migrate to the iris and retina of the eye. If the iris does not have pigment cells, it looks red (in rats and mice) or blue (dogs and cats). Odd-eyed rats are caused by the migration of pigment cells to one eye but not the other. (Note: the white coat and red eyes of albinos are not caused by a failure of pigment cell migration, but by the inability of pigment cells to produce pigment).
Pigment cells migrate to the inner ear too (cochlea and stria vascularis), where they play an undefined but essential role in maintaining hearing. If the inner ear does not have pigment cells, the individual may be deaf.
Pigment cells also migrate to the brain, to areas such as the substantia nigra (part of the midbrain that regulates mood, produces dopamine, and controls voluntary movement), the locus ceruleus (part of the brain that deals with the stress response) as well as other areas such as the leptomeninges (membranes surrounding the brain), the dorsal root ganglia, and the cranial ganglia. The failure of pigment cells to reach these areas can have a wide variety of effects, such as a movement disorder (e.g. seizures), and diverse effects on behavior and the individual's response to stress.
Pigment cells are therefore implicated in areas of the brain related to mood and the stress response. This connection between depigmentation and behavior probably played a role in animal domestication. By selecting for tameness, breeders selected for a different pigment cell migration in the developing nervous system, leading to calmer animals. A side effect of this selection for behavior was the change in pigment cell migration in the skin, leading to a piebald coat. Piebaldness and associated docility are found in many different domesticated species (horses, cows, dogs, cats, birds). In fact, selection of wild animals for tame behavior leads to depigmented areas on the fur, as shown in foxes (Belyaev 1979, Trut 1999) and rats (Trut et al. 1997). Note, however, that if depigmentation is extreme, the animal may have neurological impairments (Grandin 1998). Here's more on coat color, temperament and domestication.
Pigment cells aren't the only cells that migrate from the neural crest. Neural cells that enervate the intestines make the migration too. If the necessary neural cells don't reach the end of the intestine, the animal's intestines may not fuction properly, rendering the animal unable to pass feces, which results in megacolon.
There are many mutations that may affect the migration of cells from the neural crest. Each mutation is different, each may be modified by other genes. Each mutation may have a constellation of effects (pleiotropic effects) on coat color, behavior, and sensory function. This is why white coloration, eye color anomalies, deafness, and megacolon are often found together. They are all the result of a delay in cell migration from the neural crest.
The hooded allele
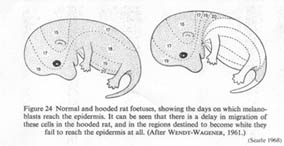
The hooded allele in the rat delays the migration of melanocytes from the neural crest (Figure 1). Consequently, the areas furthest from the dorsal midline -- feet, chest, belly -- don't have melanocytes, and those areas produce depigmented, white hair.
|
Figure 1. Normal and hooded rat fetuses, showing the days on which melanoblasts reach the epidermis. Hooded rats experience a delay in pigment cell migration (Searle 1968, from Wendt-Wagener 1961) Click on the image to get a larger view. |
- H = normal, fully pigmented coat
- h = piebald (hooded)
There are many different hooded alleles which cause different degrees of delay in melanocyte migration: e.g. h(re) (restricted, homozygous lethal), h(i) (Irish), h(n) (notch, or capped), h(e) (extreme), which produce variable amounts of depigmentation (Robinson 1989). Other genes influence the exact layout of the depigmented areas (Curtis & Dunning 1951), such as the hood modifier locus h(l) for a long dorsal stripe and h(s) for a short one.
Examples: Rats with HH have normal pigment distribution covering their entire bodies, rats with Hh are partially depigmented (white belly and pigmented sides), known as berkshire, and hh rats are hooded (pigmented head and dorsal stipe, white body).
Human analogues: The mildest analog of the hooded gene is the piebald gene in humans. Piebald humans have a white forelock, no pigment on the middle of the forehead, and other patches of depigmentation elsewhere on their bodies.
Spotting lethal and white spotting genes
Neural crest cells from the far end of the neural crest (called enteric cells) migrate to the intestine and are responsible for ennervating the colon. Normal melanocyte and enteric neural crest cell migration depends on the presence of endolethin B and endolethin B receptors, which regulate the differentiation, proliferation, and migration of melanocytes and enteric neural crest cells during development.
Many mutations may affect this progess.
Spotting lethal, a mutation in the endolethin-B receptor: A deletion in the gene for the endolethin-B receptor, (a mutation called called spotting lethal (sl)) leads to problems in melanocyte and enteric cell dispersal in rats. This leads to depigmentation of the forehead (blaze) and lack of neural connection to the colon, which means the bowel cannot be evacuated. Inability to evacuate the bowel is a condition called megacolon or megacecum, and it is fatal (Dembowski et al. 2000, Tsaur et al. 1997, Kunieda et al. 1996, Gariepy 1996, Won et al. 2002).
- Sl = normal
- sl = spotting lethal
Note: mice have a comparable mutation affecting Endolethin B, called piebald spotting (s). They also have a separate mutation that knocks out Endolethin 3, with similar effects. Confusingly, this Endolethin 3 mutation in mice is called lethal spotting (ls), which is different from spotting lethal (sl) in the rat. Whoever named these mutations needs to have his head examined.
For more on the endolethin-B receptor and megacolon in mice, see Zhu et al. 2004, and for more on mouse models of Waardenburg syndrome, see Tachibana et al. 2003.
White spotting, a mutation in the Kit protein: A separate mutation, called white spotting (Ws) knocks out the Kit protein, a tyrosine kinase transmembrane receptor, which is produced by the c-kit gene. The mutation is a 12-base deletion in the c-kit gene (Tsujimura et al. 1991). The kit protein has a wide variety of functions! Kit is involved in the development of blood stem cells (precursors to red and white blood cells), melanoblasts, and primordial germ cells, and melanoblast migration (Horie et al. 1991). So knocking out the kit protein will have a variety of effects, including: depigmentation of certain areas (up to and including entirely white with black eyes), and sometimes anemia, a deficiency of mast cells (and therefore deficiencies in histamine and serotonin), reproductive problems and deafness (Kitamura et al. 1994, Hoshino 2000, Sugimoto 1995).
These aren't the only roles of the Kit protein, however. Kit is also used in intestinal contractions: Special cells in the intestine wall, called intersticial cells of Cajal (ICC) generate electrical slow waves in the gastrointestinal tract. These slow waves regulate the frequency of intestinal muscle contractions, so ICC are critical for normal motility of the small intestine. ICC synthesize Kit, the product of c-kit. Rats with the white spotting mutation don't produce Kit in their intestines, and don't show electrical slow waves. This results in abnormal contraction and megacolon (Takeda et al. 2001).
- Ws = white spotting
- ws = normal
Note: White spotting (Ws) in the rat is similar, but not identical, to white spotting (W) in the mouse. Both are mutations in the c-kit gene.
Examples: megacolon is known to be occasionally associated with blazing in rats. Some blazed lines, such as husky (depigmented foreheads and sides, sometimes odd-eyed) may have higher incidence of megacolon.
Note, however, that megacolon may be caused by other factors as well (e.g. a separate strain of congenital megacolon of unknown cause, Lipman et al. 1998), that not all blazes are caused by the spotted lethal or white spotting genes, and that not all blazed/depigminted rats have megacolon!
Humans and other species: Humans have mutations in pigment cell migration too, called Waardenburg syndrome. There are many types of Waardenburg syndrome, each caused by a mutation in different gene (PAX3, MITF, EDNRB, EDN3 and SOX10). The most severe form of Waardenburg syndrome, called Waardenburg Type 4 or Waardenbug-Shah, includes depigmentation, eye-color anomalies, and megacolon (megacolon is called Hirschprung's disease in humans).
Endolethin B: In horses, a mutation in Endolethin B leads to Lethal White Foal Syndrome (LWFS) (Santschi et al. 1999). For more, see the Endolethin Receptor page in the Online Inheritance in Man database.
The Kit gene is implicated in the white color of some dog breeds, where it is called spotting (s). Spotting has several alleles in dogs. The dominant form (S) produces a solid coat, sometimes with a little white around the toes, chest and belly. The Irish spotting s(i) allele produces white markings on the foreface, neck, lower limbs, chest and belly (e.g. Boston Terrier and Basenji). In these animals, pigment cells never make it to the colonizing sites on the neck (Cattanach 1999).
The piebald spotting gene s(p) produces a wider distribution of white (Cocker spaniels, Pointers). The most extreme allele, s(w) is found in Dalmatians, English Setters, white Bull Terriers, and white Boxers, where it produces a white coat, sometimes with pigmented spots. In these animals, most of the coat is depigemented, but a few pigment cells may make it to the colonizing sites (notably around the eyes and ears), which proliferate and produce dark spots (Cattanach 1999).
This extreme s(w) allele is responsible for the high incidence of deafness in Dalmatians (20-30% of Dalmatians are hearing impaired -- bilaterally or unilaterally deaf). Note, however, that Dalmatians with colored patches on their ears have a lower incidence of deafness, which indicates that if pigment cells make it to the ear hearing tends to be normal (unfortunately, patches are considered a fault in Dalmatians, so breeders breed against patches, and hence are inadvertently selecting for deafness -- bummer for the dogs). Interestingly, Boston Terriers are allowed to have head patches, and they have a lower incidence of deafness (Cattanach 1999).
A mutation in the Kit gene is also responsible for the color pattern of Hereford cattle, but is not associated with deafness. Note, however, that Hereford cattle all have pigmented ears.
Mutations in melanosome formation
A problem in the lineage of cells leading to melanosomes: the red eyed dilution allele
Melanosomes are tiny little vesicles found inside the pigment cell. Pigments are assembled inside these little melanosomes, which are then transported to the edge of the pigment cell and deposit their pigment in the growing hair.
Melanosomes are actually part of a family of related "cell organs" (organelles) (Orlow 1995, 1998), that includes lysosomes and platelet dense granules. Lysosomes are little vesicles inside cells that contain enzymes involved in breaking down metabolites (waste). Platelet dense granules are found in blood platelets (they store and secrete adenosine nucleotides and serotonin). Defects in platelet dense granules lead to poor blood clotting and prolonged bleeding.
These three kinds of organelles, melanosomes, lysosomes, and platelet dense granules, all descend from a common ancestor organelle. Therefore, any mutation that affects this common ancestor will affect the descendants. The recessive red-eyed dilution mutation (r) has just this effect.
The red-eyed dilution mutation interferes with normal development of these organelles. This leads to abnormal transport of melanosomes within the pigment cell, which causes reduced pigment deposit in the hair and eyed -- hence the red eyes and pale fur. Homozygous rats with red-eyed dilution also have abnormal platelet function, called Platelet Storage Pool Deficiency (SPD) (LaVail 1981, Prieur 1984). In rats with SPD, platelets have defective secretion of clotting mediators, which leads to profuse bleeding (Raymond and Dodds 1975, Tschopp and Baumgartner 1977, Kirchmeier et al. 1990, Magro et al. 1992).
Red-eyed dilution is quite different from pink-eyed dilution, thoug the animals may have a similar appearance (though rr rats have reddish-brown eyes, while pp rats have truly pink eyes (LaVail 1981)). Genetically, however, these are quite distinct mutations that have very different effects.
Examples: An otherwise agouti rat homozygous for red-eyed dilution will be a golden tan called fawn (Prieur 1984)
Note: The red-eyed dilution of agouti isn't the only way to get a fawn colored rat. There is also a separate fawn mutation (f), which reduces pigmentation in both black and blue animals, though its cellular mechanism is unknown. Fawn on a black rat produces a coffee brown animal, while fawn on a blue animal produces a fawn animal (Castle and King 1947).
Other effects: Fawn (rr on agouti) hooded rats are used extensively in research, and have a whole list of associated disorders:
- hypertension (Rudofsky and Magro 1982, Kuijpers et al.
1986, Kuijpers and Jong 1986) leading to:
- proteinuria (protein in the urine) (Kuijpers et al. 1986, Kuijpers and Jong 1986)
- focal glomerular sclerosis (scarring of kidney tissue) (Kreisberg and Karnovsky 1978)
- kidney failure (de Keijzer et al. 1989)
- altered responsiveness of serotonergic mechanisms in the central nervous system (e.g. Gudelsky et al. 1985, Wang et al. 1988). For more details, see Hulilhan-Giglin, 1992, 1993, Chen and Lawrence 2000.
- alcoholism (Daoust et al. 1991, Overstreet et al. 1992, Rezvani et al. 2002).
- high social anxiety and low aggression (Kantor et al. 2000).
Fawn hooded rats have been used as an animal model for human psychiatric disorders involving anomalies in serotonin function, such as:
- depression (Overstreet et al. 1992, Rezvani et al. 2002)
- anxiety (Altemus 1994)
- obsessive-compulsive disorder
- eating disorders
Only the platelet storage disorder discussed above, and a serotonin uptake disorder (Tobach et al. 1984) have been shown to be caused by the red-eyed dilution gene (Hamada 1997, Fugimori et al. 1998, Prieur 1984). Many of these other disorders may be caused by other genes, which have come to be associated with these laboratory lineages of fawn rats (Overstreet and Rezvani 1996, Overstreet et al. 1999, Rezvani et al. 2002).
Human analogues: There are at least 15 mouse analogues, including: light ear, maroon, pallid, pearl, and ruby-eyed. Like red-eyed dilution, these analogous mutations affect melanosomes, platelet storage granules, and lysosomes. However, none of these analogous mutations are exactly the same mutation as red-eyed dilution. In other words, these analogues affect the same process but in different ways (Nguyen et al. 2002, Prieur 1984).
There are several human analogues that show platelet storage deficiency and depigmentation:
Hermansky-Pudlak syndrome (HPS). Individuals with HPS have a range of depigmentation, from white hair and skin to brown hair and skin due to many freckles. Individuals with HPS have decreased visual acuity and lung problems (pulmonary fibrosis) and intestinal problems (granulomatous colitis). HPS is caused by a problem in the membrane proteins of the three organelles mentioned above (melanosomes, lysosomes, and platelet storage granules) which result in defective transport. There are at least three different types of HPS (Huizing and Gahl 2002). It is rare in most human populations, but is the most common type of albinism in Puerto Rico. The mouse homologue of HPS is pale ear.
Chediak-Higashi syndrome (CHS). Humans with CHS show profound depigmentation of skin, severe recurrent infections, proliferation of lymphoid tissue (lymphoproliferative disorder) and numbness of the extremeties (progressive peripheral neuropathy). Individuals with CHS have giant melanosomes in their melanocytes and giant lysosomes in their white blood cells (leucocytes). This is a serious condition and many people with CHS die prematurely. CHS is also found in mink, cattle, mice and cats. The mouse homologue of CHS is beige. There is also at least one instance in the literature of beige rats with CHS symptoms (Ozaki et al. 1998).
Mutations in pigment transport and depositition
I. Switching between light and dark on a single hair: black and the agouti gene
Melanocytes produce two types of pigment, brown-black eumelanins and red-yellow phaeomelanins. The relative proportions of these pigments are regulated by:
(1) alpha-MSH, which binds to a melanocortin receptor called MCR1 on melanocytes and stimulates them to produce brown-black eumelanins by stimulating the production of tyrosinase, and
(2) the Agouti protein, which inhibits MSH from binding to MCR1 and results in the production of red-yellow phaeomelanins (Lu et al. 1994).
Agouti has a separate avenue of action as well: Agouti inhibits melanogenesis and generally reduces the synthesis of both pigments (Graham et al. 1997).
- A = aguoti
- a = non-agouti
So, rats with the agouti protein alternate between brown-black and red-yellow pigment production, producing banded hairs, which is the wild-type phenotype of the Norway rat. Rats without the agouti protein do not alternate. They produce eumelanins continuously and their hairs are the same dark color the length of the shaft.
Examples: Rats with aa have solid hairs (with no other modifiers, these are black rats), rats with A have banded hairs, called agouti.
Other effects: Agouti protein also prevents MSH from binding to its melanocortin receptors on neural cells (Lu et al. 1994, Willard et al. 1995). These melanocortins are potent neuromodulators that have diverse effects on mammal behavior and physiology, such that non-agouti rats are calmer and easier to handle than agouti rats (Keeler 1942, Cottle and Price 1987). The behavioral differences between agouti and nonagouti rats are probably due to the regulation of MSH and its effects on the brain and subsequent behavior.
The agouti gene therefore has an impact on behavior and may have been important in the domestication of the rat. Eighty percent of domestic laboratory rat strains are homozygous for the nonagouti allele (see Price 2002 p. 16-17 for a discussion).
An additional side effect occurs in a mutant with over-expression of agouti in mice. Agouti binds to neural melanocortin-4 receptors (MC4) in the brain, which are involved in food intake and homeostasis (Skuladottir 1999). Mice with over-expression of Agouti are yellow (from exclusive production of phaeomelanins) and obese from increased food intake.
Human analogues: Humans have an agouti analogue called agouti signal protein, ASIP, but its role is poorly defined. It is expressed most in adipose (fat) tissue where it may antagonize one of the melnocortin receptors. It may play a role in energy homeostasis and and possibly human pigmentation (Voisey & Daal 2002).
More interesting stuff about the MCR1-MSH... as you might expect, a mutation in the MCR1 receptor would have interesting consequences for pigmentation. MCR1 binds MSH, which causes the production of dark eumelanin. With a non-functional MCR1, MSH can't bind, and the melanosomes produces only light, yellow or red phaeomelanins. Mutations in extension, the gene that codes for MCR1, are not found in rats. But they are found in lots of other species, such as mice, which produces a yellow coat color (in mice, extension is called yellow (e)) (Robbins et al. 1993). Extension mutations in the horse produce the chestnut color (Marklund et al. 1996). Extension is also found in red foxes, and in dogs such as yellow labradors, golden retrievers, and Irish setters (Newton et al 2000; Everts et al. 2000).
Other mutations in MCR1 cause it to be hyperactive, binding MSH all the time and leading to the continuous production of dark eumelanin. The black of black panthers is caused by such a mutation (Robbins et al. 1993), as is the black of black sheep (Vage et al. 1999). Hyperactive MSH mutations are found in mice too, in the form of somber and tobacco darkening alleles (Robbins et al. 1993).
MCR1 varies seasonally in some species, which is what causes the transition from pale winter coats to dark spring and summer coats in some animals.
In humans, mutations in the MCR1 receptors cause melanocytes to synthesize red-yellow phaeomelanin, which produces red hair. There are 6 known mutations in MCR1 which vary in how much they knock out MCR1's function. Red-haired individuals are usually homozygotes or compound heterozygoes (posessing two different types of MCR1 mutation). Two alleles for non-functional MCR1 will produce bright red hair. People with just one mutated MCR1 allele, or alleles that only partly impair MCR1's function, may show varying shades of red, or no red at all. About 50% of white people carry a mutated MCR1, which is thought to be significantly associated with fair skin even if it does not produce red hair (Ha and Rees 2001, Rees 2000, Schaffer and Bologna 2001).
As it turns out, phaeomelanins aren't as good at protecting the skin from ultraviolet rays as eumelanins are, which is why red-headed people are more prone to skin cancer.
- For more on red hair: Red Hair Genetics
II. Less pigment deposited in the hair results in a lighter coat: the dilute gene
Once the pigments are made, they have to be transported to the hair. The pigment particles, called granules, are synthesized in little vesicles called melanosomes, which are transported via the dendrites of the melanocyte to the shaft of the growing hair. The transport is actually really neat... melanosomes are carried by little molecular feet (myosin 5, an actin dependent motor protein) along little branching molecular "ladders" (actin filaments) contained in projections off the melanocyte (dendrites) to the cell's edge. If this transportation system is affected, transport to the cell edge isn't normal.
Rats with the dilute mutation (called Myo5a(d) in the literature) produce normal types and quantities of pigment in their melanocytes, but the transport of these pigments to the hair shaft is disrupted due to a mutation in myosin 5. Many melanosomes with their pigments are stuck in the cell center, unable to be transported out to the cell edge. So less pigment is incorporated in the hair, and when it is incorporated it tends to be deposited in clumps. This gives the coat a washed out, diluted color (Wu et al. 1998, Wei et al. 1997).
- d (also called MyoVa, Myo5a) = dilute
- D = normal
There are several alleles of the dilute gene, such as dilute lethal and dilute opisthotonus (dop), which result in severe neurological defects (ataxia, seizures) and sometimes death in the homozygous state (Futaki et al. 2000; Ohno et al., 1996; Dekker-Ohno 1993).
Examples: Rats which are genetically black but have the dilute mutation are a slate grey called blue.
Note, however, that there are many different mutations that give rise to blue rats in the pet rat fancy, so it is not clear which pet rat mutation (if any) corresponds to the known mutation in MyoVa in laboratory rats. Also note that the names of the different shades of blue in pet rats and the Mendelian notations used to designate them among rat breeders may differ between pet rat organizations and countries.
Other effects: Pigment transport isn't the only thing affected when myosin 5 is impaired. Myosin 5 is also used in the elaboration and maintenance of cellular processes of the melanocytes: homozygous d rats have melanocytes with fewer and thinner dendritic processes. Myosin 5 is also responsible for dragging endoplasmic reticulum (ER) to the cell edge of Purkinje cells (large, important neurons in the brain) where it produces calcium fluxes. If myosin 5 is disrupted, it can't drag ER to the cell edge, calcium fluxes are disrupted, which reduces the Purkinje cell's excitability, which results in neurological deficits as seen in some of the more severe dilute mutations (see Tab et al. 1998; Takagishi 1998; Hurvitz et al. 1993).
Human analogues: The human counterpart of the dilute mutation is found in Griscelli Prunieras syndrome, a rare autosomal recessive disease (Westbroek et al 2001). There are two types. Individuals with Griscelli type 1 have a mutation in myosin 5 and are characterized by partial albinism, silver-blond hair discoloration, and neurological deficits. Individuals with Griscelli type 2 have a mutation in another transport protein, called Rab27a, and they have the symptoms of type 1 plus primary immunodeficiency (Klein et al. 1994). Melanocytes of people with Griscelli symdromes have short, stubby dendrites, and contain mature melanosomes, indicating a transfer block toward the surrounding keratinocytes.
Mutations in pigment synthesis
I. A monkey wrench right at the start: the Chinchilla mutations
One of the earliest mutations in the pigment production pathway is a mutation in the enzyme tyrosinase, which converts the pigment precursor tyrosine into the next step. The gene for tyrosinase is named chinchilla. If a mutation in the chinchilla gene results in a completely nonfunctional tyrosinase enzyme, then the animal will be unable to produce any pigment anywhere in the body. Animals with this mutation are called albinos.
The chinchilla gene has other known mutations, however, and many of them result in a semi-functional tyrosinase. These produce animals with diluted color compared to those with normal tyrosinase. In the acromelanic version, the semi-functional tyrosinase is very fragile and temperature dependent. Raise the temperature too much and the tyrosinase breaks. Rats and other animals with temperature-sensitive tyrosinase only produce pigment in the cooler areas of their bodies: the extremeties such as nose, ears, feet and tail. Animals with these temperature-sensitive mutations are siamese or himalayan. For a specific molecular description of how the tyrosinase produced by the achromelanic mutation differs from the normal tyrosinase, see Kwon et al. 1989.
Here are some of the mutations in the chinchilla gene for tyrosinase:
- C = full color
- C(ch) = Chinchilla
- c(h) = Acromelanism (siamese)
- c = Albino
Examples: C(ch)C(ch) produces chinchilla, but this is (as far as I know) unheard of in rats. Rats with cc are albino, and c(h)c(h) rats are known as siamese. The combination c(h)c produces pigmentation intermediate between albino and siamese and is known as himalayan.
Other effects: lack of melanin pigment in the eye and optic nerves leads to abnormal development and function, and hence poor vision and sometimes blindness (Balkema and Drager 1991). Absence of the fovea, nystagmus, strabismus, and reduced visual acuity are common in all types of albinism. For more on how albino rats see the world, see article entitled "what do rats see?"
Human analogues:albinism occurs in humans, too, where it is called oculocutaneous albinism type 1 (OCA1). There are about 60 mutations that affect the the tyrosinase gene in humans (Oetting & King 1993, Spritz 1994). How much pigment individuals produce depends on how impared the tyrosinase enzyme is -- pigment expression can range from completely absent (white skin, white hair, and depigmented eyes) to nearly normal.
There are also human analogues of the himalayan or siamese mutation, a variant of the oculocutaneous albinism type 1 (OCA1). Like siamese animals, humans with temperature sensitive tyrosinase produce white hair in the warm areas of their bodies and darker hair in the cooler areas. They have white axillary (armpit) hair, nearly white scalp hair, light brown arm hair and dark brown leg hair, and no generalized skin pigment (King et al. 1991; Giebel et al. 1991; Oetting & King, the clinical spectrum of albinism in humans,).
- More on oculocutaneous albinism in humans
- Also see Boissy 1997.
II. A poor cellular environment for pigment synthesis: the pink-eyed dilution mutation
Pigment synthesis occurs in melanosomes: Tyrosine enters the melanosome and the enzyme tyrosinase catalyzes it into dopaquinone. Later on, down the eumelanin pathway, tyrosinase-related-proteins turn brown pigment into black eumelanins. These chemical reactions can be enhanced or impaired by changes in the internal pH of the melanosome.
The p locus codes for a protein located on the eumelanosome's membrane, a gate (transporter protein) that lets molecules into the cell. The gate appears to help regulate the pH of the melanosome by letting anions in (a different gate admits H+). A mutation in the p locus affects this gate, changing the normal, acidic pH to a more neutral environment. As p proteins are not found on phaeomelanosomes, phaeomelanin production is not affected by mutations in p.
An acidic environment in the melanosome is required for normal tyrosinase activity (Brilliant et al. 2001. Strum 2001. Puri et al. 2000). When melanosomes are acidic, they produce more pigment, with a preferential increase in the dark eumelanins. When they are neutral, they produce less eumelanin, but phaeomelanin is relatively unaffected (Brilliant 2001). There is also some question of the p gene being a tyrosine transporter (Rinchik 1993), but the evidence is mixed.
So the p allele dilutes whatever color it is found with, by reducing black-brown pigments and leaving the red-yellows alone. The net result, in rats, is reduction in the dark eumelanin pigments, resulting in a pale coat and pink eyes.
The p mutation has been found to be a deletion including exons 17 and 18. It is an old mutation that is shared between a number of laboratory rat strains (Kuramoto et al. 2005.)
- P = normal pigmentation
- p = pink-eyed dilute
Examples: in the agouti rat, the pink-eyed dilution dilutes the dark band on each hair to a pale color, resulting in an overall combination sometimes called amber. Straight black is diluted to a pale yellow which is sometimes called champagne.
Other effects: few, abnormally shaped melanosomes.
Human analogue: Normal variation in the human p locus causes normal skin color variation among different ethnic groups.
The strong effect of the rat's pink-eye dilution p mutation has its human analog in oculocutaneous albinism type 2, (OCA2). OCA 2 is the most common type of albinism in humans, especially among people of African descent. Characeristics are highly variable, however, ranging from minimal to moderate pigmentation of the hair, skin, and iris. The pigment may be localized in freckles. Individuals of African descent with OCA2 tend to have yellow hair, white skin with localized pigment regions, and irises that are partially or completely pigmented with a tan melanin. The p gene in humans is located on a segment of chromosome 15, which is deleted in people with Prader-Willi and Angelman syndromes, which may explain why individuals with these syndromes have hypopigmented hair, eyes and skin (Rinchik et al. 1993, Brilliant et al. 1994).
III. A last step in pigment production that doesn't happen: the brown mutation
After dopaquinone, the pigment pathway splits into two. One branch leads to eumelanin and the other leads to phaeomelanin. One of the mutations that affects the production of eumelanin is a mutation in the brown gene, which codes for the enzyme TYRP1, which catalyzes the final step of eumelanin synthesis: converting brown pigment to black. With normal TYRP1, the final step takes place and the animals produce black eumelanin. A mutation in TYRP1 means this final step cannot happen, and animals with this mutation produce brown eumelanin. There are at least two types of TYRP1 mutations: one is a point mutation in TYRP1 which renders it non-functional, the other is a mutation elsewhere that causes TYRP1 to be produced in low quantities.
- B = black
- b = brown (also known as TYRP1(b))
Examples: Rats homozygous for the brown mutation are called chocolate, in dogs this is often called liver. Agouti rats homozygous for brown have brown instead of black eumelanin stripes on their hairs, and are sometimes called chocolate agouti.
Other effects: TYRP1 mutations also affect cell proliferation and melanosomal maturation (Sarangarajan et al. 2000).
Human analogues: mutations in TYRP1 are found in humans, too, where they produces oculocutaneous albinism type 3 (OCA3). In humans as in mice and rats, TYRP1 catalyzes eumelanin synthesis, but in humans it may have additional roles, such as maintaining the stability of tyrosinase and modulating its activity. It also maintainins melanosome structure and affects melanocyte proliferation and cell death (Sarangarajan & Boissy 2001, Sarangarajan et al. 2000).
Oculocutaneous albinism Type 3, also known as rufous oculocutaneous albinism, xanthism, or xanthous albinism, is a common autosomal disorder found in blacks (Manga 1997). People with rufous albinism produce normal phaeomelanins (reds and yellows) but do not produce the black eumelanins, only the brown ones. The first description of a person with xanthous albinism was of an African-American twin boy who had light brown skin, light brown hair, and blue/gray irises while his fraternal twin had normal pigmentation. More individuals have been described since then, such individuals have a red to reddish brown coloration of the skin and hair and reddish brown, slightly translucent irises; photophobia and nystagmus are mild and visual acuity is normal or nearly normal.
Online resources: